
Longread
Quick and simple gene repairs
It is a dream solution for molecular biological research. The CRISPR-Cas9 technique allows changes to be made in DNA quickly and cheaply. ‘Hopefully, it will very soon be possible to cure people. Wouldn’t that be fantastic?’
The CRISPR-Cas gene editing technique lets a selected gene be cut very precisely or replaced by another gene.
A rolled-up piece of paper is pinned to the noticeboard in Professor John van der Oost’s room. It is evidence of a bet. Will the scientists who developed the gene technique CRISPR-Cas9 be awarded a Nobel Prize in the years to come (something no one really doubts) and which researchers will be given the honour? Van der Oost and a postdoc have bet a bottle of wine on the outcome.
No, says Van der Oost, he didn't bet on himself. In the Dutch newspaper NRC, cancer researcher and former columnist Piet Borst wrote that Van der Oost deserves a Nobel Prize for his role in the development of CRISPR-Cas9. ‘That was nice to read, good to see someone giving us the credit for all the fundamental work that we carried out. But as Borst himself said, the prize for medicine won't go to a microbiologist.’ In scientific journals, Van der Oost is indeed seen as the pioneer paving the way for the three researchers judged likely to get the prize: the French scientist Emmanuelle Charpentier and the Americans Jennifer Doudna (Berkeley) and Feng Zhang (MIT).The triumphant march of this new technique, which lets precise changes be made in DNA at low cost, started in the late 1990s quite by accident in Wageningen.
‘We were involved in deciphering a genome from a bacterium from a geyser in Yellowstone,’ says Van der Oost. ‘We found that it contained repeating pieces of DNA. We published this result in 2001 and then forgot about it. We had no idea what it was for. In 2005 French and Spanish researchers suggested that this could be an immune system to protect bacteria from viruses. I had just received a Vici grant from the Netherlands Organization for Scientific Research and that's what made the difference. It let us immediately put a postdoc and a PhD candidate to work on the topic.’
Invading viruses
That led to an article in Science in 2008 in which the Wageningen group explains how the immune system works to protect the bacterium E. coli from invading viruses. To do this, the bacterium has incorporated fragments of DNA from the attackers. Copies of this (CRISPR-RNA) patrol the cell. If this CRISPR-RNA recognizes a virus that has got in, it squeezes between the two DNA strands of the virus and pushes them apart. Then special proteins known as Cas inflict the mortal blow: they cut the viral DNA to shreds. That is the end of the invader. The Wageningen study also showed that the CRISPR-RNA can be adapted so that the protein scissors cut where you want them to in the DNA. The publication led to a deluge of research on variants of this immune system. The now-celebrated CRISPR-Cas9, which was discovered in lactic acid bacteria, is a simpler variant than the system Van der Oost discovered in E. coli. It has turned out to be just the thing for a wide range of applications in medical and biotechnological labs. That is because the cutting mechanism, the protein scissors that bacteria use to protect themselves against viruses, also works in plant, animal and human cells. It can be used in these cells for gene editing, rewriting DNA both very precisely and quickly. But you also need something to tell the protein scissors where to cut. American researchers managed to give the scissors a different guide every time that took them to just the right spot in the genetic material.
That made gene editing virtually a piece of cake. The researcher decides where they want to make a change in the DNA of the organism they are studying. Then they order a CRISPR-Cas9 set online from a supplier that will contain the piece of RNA that corresponds to the location in question on the DNA. Once the postal service has delivered the set, it's child's play for an experienced laboratory technician to insert it in the cell nucleus. Then the RNA – the guide – will take the scissors along in search of the spot in the genetic material where the cutting is required.
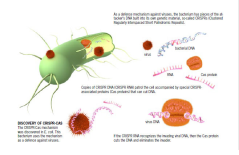
Deactivating genes
Cutting sounds destructive but sometimes that is all that is needed to deactivate a bad gene. But making repairs is not always that simple. Sometimes the CRISPR-Cas9 set has to include the good gene as well. The cell uses that repair set to rectify the original error. Last summer, a team of American, Chinese and Korean researchers published an article on how they were able to use this technology to remove the hereditary heart condition hypertrophic cardiomyopathy from human embryos. A tiny but destructive genetic abnormality was replaced very accurately with a healthy piece of DNA. It should be noted that this was an experiment in the laboratory; the use of genetically modified embryos is currently forbidden. Were such an embryo to be placed in the uterus, it would very probably result in a healthy child. Researchers can go one step further still by having repair sets with an extra gene to introduce new properties. British scientists used this approach to treat a baby suffering from an incurable form of leukaemia. They used CRISPR-Cas9 to programme the immune cells to attack the cancerous cells. But the possible applications are much broader than just medicine. The precision instrument CRISPR-Cas9 is a dream solution for all kinds of molecular biological research. Older techniques for modifying DNA are not nearly as accurate and require far more effort. What now takes a couple of weeks used to cost two to six months and the result was less predictable.
Gene therapy in humans
Despite this precision, even CRISPR-Cas9 makes the occasional blunder, for example by snipping in the wrong place. ‘If that happens to be in a different gene, you might even end up further from your goal’, says Van der Oost. ‘If we want to use this for gene therapy in humans, we need to develop it further to make it error-free. Although a huge amount of progress has already been made on this front in the past few years.’
For example, when repairing the hereditary heart disease hypertrophic cardiomyopathy, the scissors did their job in the right place every time. Even so, the scientists said in their article that more research is needed before this form of gene editing can be used in hospitals. They also noted that the ethical aspects need to be discussed in a broad public debate. How far do we want to go in modifying human genes? That question is all the more urgent because the technique is so easy to apply. Should we decide on the limits first and only then proceed to further research? Van der Oost thinks that this can be done in parallel. ‘We should definitely have a debate on how far we want to go, but in practice there are already strict safeguards such as the rules on the use of embryos. People always go on about whether you should be able to turn brown eyes blue. If people with loads of money ever want to do such stupid things, we won't be able to stop them anyway. We should focus on the positive side. Just think of the opportunity we will have to eradicate a hereditary disease that has been passed down in a family! What began 10 years ago with us deciphering a defence mechanism in bacteria will hopefully very soon make it possible to cure people. Wouldn’t that be fantastic?’
Patents
The successor to the gene technique CRISPR-Cas9 is already in the making. Last year, the Wageningen microbiologist John van der Oost and Feng Zhang of the Broad Institute at MIT described and patented an alternative mechanism for cutting DNA: CRISPR-Cpf1, also known as CRISPR-Cas12. ‘Waiting to see how successful it becomes is exciting,’ says Van der Oost. ‘Income is already beginning to trickle in. It has been agreed that the money that Wageningen University receives will be used to encourage innovative research in biotechnology.’
Feng Zhang has been involved in a fierce legal battle ever since 2013 about the main patent for CRISPR-Cas9. It was assigned to Zhang but that decision has been challenged by Emmanuelle Charpentier and Jennifer Doudna. So far the decision has been upheld by the courts. Experts speculate that the patent could be worth hundreds of millions of dollars.
Verifying mutations
CRISPR-Cas is now a familiar technique among other chair groups in Wageningen too. ‘It's an important tool in our research,’ says Martien Groenen, professor of Animal Breeding and Genetics, ‘for example to determine what a certain gene does. You can use gene editing to switch it off and then see what happens. We are currently using the technique to verify the effects of a mutation in a gene in chickens by testing it out in zebrafish. It has the same gene but it is a much easier lab animal.’
Groenen thinks CRISPR-Cas could eventually be used in animal breeding but he does see a downside. ‘In animal breeding, it's all about finding the right balance between different properties such as the milk yield, growth and resistance to disease. Focusing on one change often means you lose out in other areas. If you want to introduce a new property within a reasonable period without causing inbreeding, you have to modify thousands of animals so as not to lose any other properties.’
Ripening tomatoes
Researcher Ruud de Maagd at Wageningen Plant Research studies which genes are important for the ripening and shelf life of tomatoes and how they are regulated. Not only does he switch genes off with CRISPR-Cas, he sometimes also makes two cuts in a single DNA strand. The plant cell often glues the two ends together so that the piece between the two cuts has been removed. That provides information about how the genes are controlled by the surrounding DNA. The researcher thinks this information could eventually lead to a tomato that does not become soft so quickly and also tastes good, with a bit of genetic help from CRISPR-Cas. The big question for plant breeding companies is whether such a tomato would be allowed under the strict rules for the admission of genetically modified crops in the EU.
The EU had promised to take a decision about CRISPR-Cas in its GMO rules by the end of 2016, but the European Commission has twice delayed making that decision. French scientists have now instituted proceedings with the European Court of Justice. ‘I can understand that people are worried about playing around with DNA,’ says De Maagd. ‘But the question is whether CRISPR-Cas is always covered by the restrictions in the GMO regulations. If you just switch a gene off or remove some DNA, that change is no different from the mutations that occur in nature as well.’
More information about CRISPRCas? Read the dossier:

Author: Rik Nijland | Illustration: Maartje Kunen
This is an article from Wageningen World. Read more: www.wur.nl/en/wageningenworld
